PJ4018 Regulatory Report Assignment Sample
Here’s the best sample of PJ4018 Regulatory Report Assignment, written by the expert.
Name of the medicine is DDD-003. The medicine is used for the treatment of diabetic patients. Diabetes mellitus can be reduced by the application of medicine. The medicine is new for the market. The company brings the medicine to the market to provide treatment against the very dangerous disease diabetes. This may lead the healthcare system of the country to a more bright and more successful state. The entire study contains information on the implementation of the medicine in the market with proper testing and trials. Other sectors related to medicine were also discussed in the entire study by the researchers.
General investigation plan
The investigation and testing for an upcoming medicine are very important. The effectiveness and issues of the medicine can be focused on by this kind of investigation. Not only this, but the investigation is also responsible to evaluate the limitation of the medicine and or provide more effort to make this appropriate (Tran et al. 2020). A proper investigation is also related to the stability and storage condition of the medicine as well. The medicine DDD-003 is an upcoming product that will be able to help people who are suffering from the terrible diseases of diabetes. A simple carelessness of the investigators may lead the patient’s life into a very critical condition.
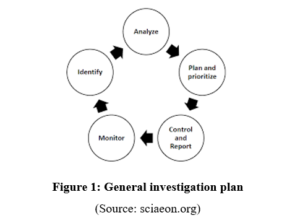
For that reason, the investigation process of the medicine would have conducted witty proper process and actions. The investigation process had been conducted with some different phases. All the phases are conducted with maintaining safety rules and precautions properly. The primary phase is as important as the final phase. The phases are focused on a wide range of use of the upcoming medicine (Goval et al. 2018). The entire investigation plan is based on the effectiveness of the medicine for diabetes mellitus. The limitation or lack of effectiveness can also be expressed by the investigation plan of the scholars. The compact findings of all the phases are responsible for successfully launching the DDD-003 medicine to the market for diabetic patients. All the facts and perspectives of the investigation are mentioned in the entire assignment with appropriate points.
Toxicology and dosage
Treatments for diabetic mellitus are diverse, having vastly disparate safety profiles in therapy usage and overdose. DDD-003 can be severe and last for a long time if patients take too much insulin. If feasible, sulfonylurea toxicity should be managed with docetaxel rather than intravenous insulin. Rapid fructose overdose can lead to life acidosis with high lactate levels, necessitating treatment. The appropriate dosage of the medicine is considered to 500-1000 mg/m² for the patients.
Investigator Brochure
Diabetes mellitus is a chronic condition marked by hyperglycemia as a result of impaired insulin production, insulin sensitivity, or both. Insulin resistance is linked to substantial long-term consequences, including damage, malfunction, and collapse of different organs, including the kidneys, eyes, nerve, brain, and capillaries. Prediabetes is a multifaceted condition characterized by reduced beta-cell activity, insulin sensitivity resistance, and impaired hepatic glucose metabolism in varying degrees. Insulin-dependent patients’ blood sugar control may decrease with time (Tigga and Garg, 2020). Hormonal glargine DDD-003 is a once-daily lengthy medication analog used to cure diabetes mellitus when used in conjunction with oral hypoglycaemic medicines and as a component of a systemic drug program. The medicine DDD-003 is a commonly used baseline treatment across the world, hence it was chosen as the referent in this study. The investigation of medicine is based on the various perspectives of the diseases. The effectiveness and limitation of the use of DDD-003 is also a part of the entire investigation conducted by the researchers before launching the product to the market.
Protocols
Phase I: The first trial of the medicine belongs to the process of “investigational new drug application (INDA)”. During this phase, the researchers test the medicine on some human being in the lab. They are engaged into the testing with their own concern and responsibility to bring a huge revolution in the treatment of diabetes mellitus (Kullar et al. 2018). This phase was all about testing the level of the components while maintaining the fact of “Common Technical Document (CTD)”. This phase is like the first step for launching the medicine into the market. Getting success into this phase can be able to increase the focus of the researchers for the entire investigation process. Achieving success in this step is also responsible for applying medicine to the living being.

Phase II: This phase also belongs to the process of INDA. During this phase, the researchers to apply medicine on them have used some living creatures. During this phase rats, mice and dogs had been used by the researchers for measuring the efficiency of the medicine on the living creature (Benamina et al. 2018). This phase had been conducted with the help of toxicological studies for using the lying being in the research work. Achieving success in this phase can help the researchers to apply the medicine to the human body also. This phase is also very important for the investigation method of medicine.
Phase III: This phase is considered the final step for launching the medicine into the market. During this phase, the medicine was applied to the human body for measuring the result of it. This phase had been conducted by maintaining ICH guidelines with the help of “Chemistry Manufacturing and Controls (CMC)” (Avtanski et al. 2019). By conducting this phase the entire investigating protocol of the company may have been completed and they are capable of launching their medicine successfully to the market. The success of the final phase is the most essential fact for the entire investigation process.
Chemical Manufacture Control (CMC) data
Provide up-to-date legal knowledge on intentions, antecedents, and prospective competitors, as well as knowledge on relevant governing needs. Consult with local regulatory agencies. Facilitate the effective oversight and reporting of documents. Labeling, anecdotal evidence and clinical studies, and CMC are all areas where you can provide specialized functional knowledge (Li et al. 2019). Any regulatory strategy’s overarching goal is to ensure that patients have access to life-saving medications. It’s simply one part of the new “Drug Application marketing application (NDA)”, and a CMC regulatory specialist is needed to create proper CMC normative arguments in this context. The plan must address regulation CMC goals, impediments, the enable stakeholder’s environment, and any applicable antecedents, as well as critical CMC benchmarks and process steps. It must also investigate and identify any possible hazards to achieving a certain regulatory goal. Throughout the item’s lifespan, from development for the treatment to article operations, drug suppliers must have a considerable say in the development strategy (Xing et al. 2020). The adopted CMC is also capable to focus on the effectiveness and lifetime of the medicine of DDD-003. The components are also evaluated with the help of the techniques adopted by the researchers during the investigation. There is brief information about all the perspectives of medicine for the treatment of diabetic patients throughout the country. All the processes are maintained by the safety and regularity protocol set by the medical authority of the country.
Stability and storage conditions
The effect of the medicine stays for almost 5 to 8 hours among the dogs and tars. These findings also belong to the process of investigation held by the research team during their work. For the human being, the effect of the medicines lasts almost 72 hours approximately. Appropriate storage is a very important factor for this medicine. The medicine should be kept in a cold and dry place. This place also should be dark and far from the sunlight.
Special considerations
The medicine is associated with the treatment of the chronic disease of diabetes. The disease is a very problematic issue for many people of the nation. Launching the medicine into the market helps the patients to stay a healthy and happy life. For this reason, the research team has conducted the entire study or research work. By conducting this research work, the researchers can create a huge effort to reduce diabetes among people (Due-Christensen et al. 2018). All the processes of implementation and investigation phases are also very important for launching the product into the market. This fact can play a very precious role in helping patients who are suffering from diabetes for many years.
Conclusion
The entire study is about the launching of the medicine DDD-003 for the treatment of chronic disease diabetes. The drug is a brand-new product on the market. The business is bringing the pharmaceutical to market to treat diabetes, which is a highly deadly condition. This might lead to a brighter and more prosperous future for the country’s healthcare system. The complete report includes information on putting the drug on the market and doing proper testing and trials. The researchers also explored other aspects of medicine during the investigation. In conclusion, it can be said that the company considers the entire study as compact information for launching the new medicine to the market.
Reference List
Journals
Avtanski, D., Pavlov, V.A., Tracey, K.J. and Poretsky, L., 2019. Characterization of inflammation and insulin resistance in high‐fat diet‐induced male C57BL/6J mouse model of obesity. Animal models and experimental medicine, 2(4), pp.252-258.
Benamina, M., Atmani, B. and Benbelkacem, S., 2018. Diabetes diagnosis by case-based reasoning and fuzzy logic. IJIMAI, 5(3), pp.72-80.
Due-Christensen, M., Zoffmann, V., Willaing, I., Hopkins, D. and Forbes, A., 2018. The process of adaptation following a new diagnosis of type 1 diabetes in adulthood: a meta-synthesis. Qualitative health research, 28(2), pp.245-258.
Goyal, M., Reeves, N.D., Davison, A.K., Rajbhandari, S., Spragg, J. and Yap, M.H., 2018. Dfunet: Convolutional neural networks for diabetic foot ulcer classification. IEEE Transactions on Emerging Topics in Computational Intelligence, 4(5), pp.728-739.
Khullar, D., Wolfson, D. and Casalino, L.P., 2018. Professionalism, performance, and the future of physician incentives. Jama, 320(23), pp.2419-2420.
Li, L., Xu, M., Wang, X., Jiang, L. and Liu, H., 2019. Attention based glaucoma detection: a large-scale database and CNN model. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition (pp. 10571-10580).
Tran, N., Pham, B. and Le, L., 2020. Bioactive compounds in anti-diabetic plants: From herbal medicine to modern drug discovery. Biology, 9(9), p.252.
Xing, B., Zhao, Y., Dong, B., Zhou, Y., Lv, W. and Zhao, W., 2020. Effects of sodium–glucose cotransporter 2 inhibitors on non‐alcoholic fatty liver disease in patients with type 2 diabetes: a meta‐analysis of randomized controlled trials. Journal of diabetes investigation, 11(5), pp.1238-1247.
________________________________________________________________________________
Know more about UniqueSubmission’s other writing services:

