- Posted on
- Unique Submission Team
Advanved Materials
The material of aluminum silicate is a composition of half amount silicon oxide and alumina; it is also named as M120F. As commented by Carrera et al. (2016, p.553), sheets of aluminum silica consists of high purity aluminum oxide, which are available in nature with number of standard thickness and dimension.
This material has a good feature as thermal shock resistant; therefore it is easily replaceable at carbon fixtures. This versatile composite material can also be tailored through changing the process conditions. In order to provide hardness values as per customer requirements, this material is used.
An abundance and variety of many silicate materials can be seen in nature. These are the result of significant nature of silicate atom. Napoletano et al. (2018, p.211) has asserted that the stability and versatility and stability are notable, when it makes bonding with oxygen atom. Silicate anion (noted by (SiO4)4- ) takes a shape of tetrahedron. Here, Si4+ places at centre of tetrahedron and four anions O2- place at four corners.
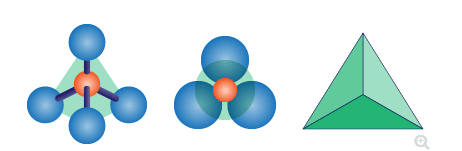
Figure 1: Structure of Aluminium silicate
(Source: Influenced by Senthamizhan et al. 2017, p.39)
Within the tetrahedron structure, all Si-O bond are partially covalent and partially ionic, therefore they are strong. Bonds of aluminum silica connected with each other in different ways according to the number of cations. About 25 out of 100 silicate materials are common.
From XRD images of aluminum silicate, it can be implied that there is a strong bond between any other crystal and silicate. Krutpijit and Jongsomjit (2016, p.353) has stated that in silica tetrahedron, every Si-O covalent bond requires the half of number of electrons in O2- ions.
It means that each O2- ion is connected with second ion and a silicate ion. Consequently, it can be polymerized in the form of chain. For this reason, alumino silicate sheets always stick together.
Montmorillonite clay falls in a group of soft phyllosilicate minerals (Kadoura et al. 2016, p.339). It forms by water precipitation of various microscopic crystals, which are known as natural clay. Gallery spaces of Montmorillonite clay are mentioned as below.
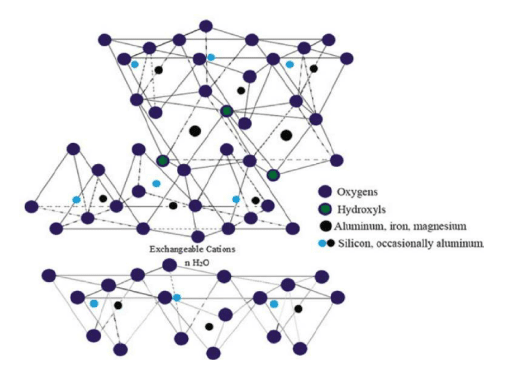
Figure 2: Gallery spaces of Montmorillonite clay
(Source: Inspired by Teich-McGoldrick et al. 2015, p.20881)
This clay is composed by metal oxides, hydroxides as well as water trapped oxides and silicates (Demers et al. 2017, p.00679). Moreover, it is structured by the polar attraction of ions. It can be obtained by following materials.
- (Ca0.12 Na0.32 K0.05 ) [Al3.01 Fe(III)0.41 Mg0.54] [Si7.98 Al0.02] O20 (OH)4
- CEC (Cation Exchange Capacity) of almost 85+/- 3 mmolc/100g
- Layer charge ion of almost 0.32eq/ mol within one (Si, Al) 4 O10
- ESA (External Surface Area) of almost 23m2/gm
- Mean size of particle 3.2 µm
- The range of 3˜ 10 µm between d25 and d75 size
Swelling nature of a nanocomposite hydrogel has network structure of inorganic clay and organic polymer. Through focusing on role of nanocomposite hydrogel and exfoliated clay, these network structures can be investigated.
Moreover, in opinion of Dos Santos et al. (2018, p.138), sodium counterions are responsible for swelling conditions of clay. Within water, NC clays exhibit swelling and deswelling behavior. For adding solvent, polymer obtains more swelling property. In this context, swelling property depend on two primary factors.
- App for Polymer Solubility is controlled by X parameters and Flory-Huggins.
- Length of chain and its crosslinks is responsible for swelling property. Shorter length implies higher crosslink density and less swelling.
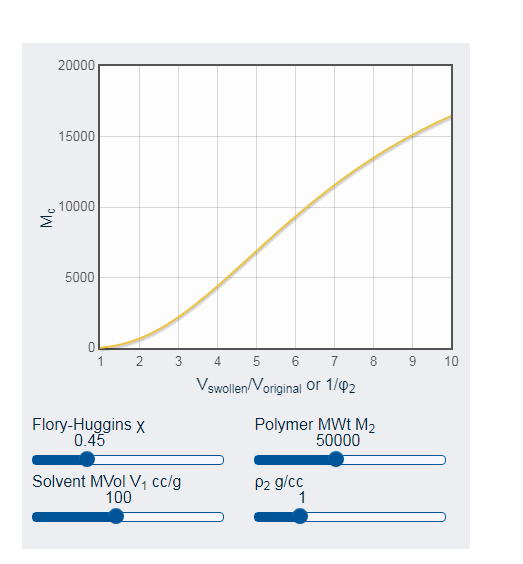
Figure 3: Length of chain and its crosslinks
(Source: Influenced by McCombe et al. 2016, p.455)
Method of exfoliation of clay by polymer has mentioned in figure.
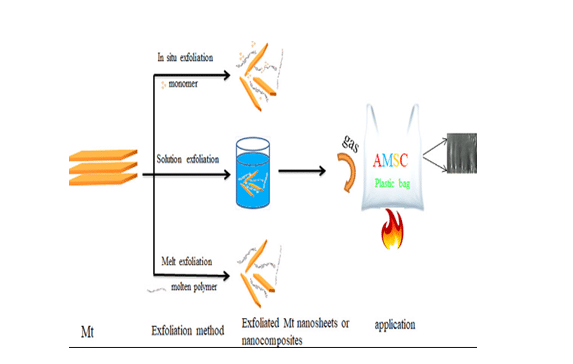
Figure 4: Exfoliation of clay
(Source: Influenced by HODAEI and FARHANG, 2015, p.00443)
Direct exfoliation of Mt clay can be done by water dispersion with organic solvents (Demers et al. 2017, p.00676). It can be further intensified by method of ultrasonication. At first, Mt clay needs to grind in solid form within high energy ball. At mill, this clay can be directly exfoiled into some extent. Three stages of clay exfoliation are solution, melt and situ exfoliation.
Some of the below mentioned ions are used to promote clay exfoliation.
- Modified MMT, whose trade name Dellite ® 67G and di (hydrogenated tallow) of 40 wt %
- 2HT dimethyl ammonium from Laviosa Chimica Mineraria S. P. A
The materials of sPS are used in the gel permeation chromatography. This is produced by Dow chemicals. Over 98% syndiotactic triads content, 13C structure can be used to categorize magnetic resonance.
Additionally, McCombe et al. (2016, p.455) has narrated that average molar mass of clay is obtained by GPC method with trichloro benzene at the temperature 135C. Consequently, it contributes 3.9 Mw / Mn index into clay.
Alumina silicate sheets help to improve theoretical strength of any material as it increases chemical durability and mechanical property. Consequently, it has huge technical and industrial applications. Dynamic fatigue nature of alumina silicate enhances the tendency of ion exchange.
As commented by Krutpijit and Jongsomjit (2016, p.353), ability and compressive characteristics of surface help to delay crack growth. According to age, bulk stresses also increases its magnitude. Therefore, dynamic fatigue and ion exchange behavior are responsible for closeness.
Molecular structure, temperature and composition are responsible for reduction of tensile strength. Some external conditions are there to reduce hole in alumina silicate sheets.
- Air pressure, temperature, clarity and humidity of air, atmospheric refraction and wind force
- Error in instrument
- Backwardness method of instrument and imperfection causes (Carrera et al. 2016, p.553)
Solution-grown germanium nanowires are tested in anodes of high capacity. This is needed to be placed in lithium ion batteries. Films of nanowire materials are formulated, after that, it is cast as slurries.
Casting of nanowires is done by using conductive carbon named as 7:1 Ge :C w/w. The 1.0 M LiPF6 and PVdF are used to dissolve this wire within many solvents, it acts as an electrolyte. In this context, addition of some FEC (Fluoroethylene Carbonate) increases its electrolytic nature.
This function is critical to achieve a stable battery with reversible capacity. Capability of this battery after 100 number of cycles is 1248 mA h g-1. This amount is nearly close to theoretical capacity, 1384 mA h g-1 (Teich-McGoldrick et al. 2015, p.20891). For the consequence, germanium nanowire exhibits a high rated capacity.
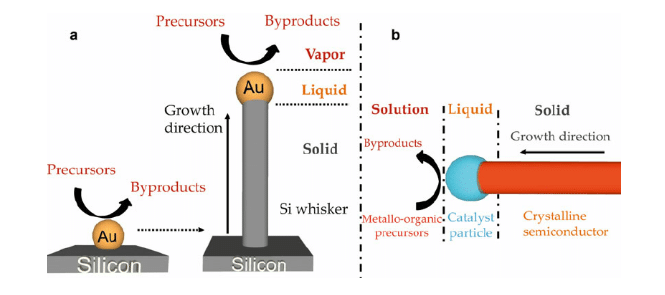
Figure 5: VLS method for nanowire germanium
(Source: HODAEI and FARHANG, 2015, p.14)
Through VLS method, it can be produced, as it helps to increase super saturation condition and decrease equilibrium concentration of Ge.
There are two different types of growth of germanium nanowires.
At the higher temperature, 345°C to 375°C mass transport occurs in germanium precursor (according to kinetics data), this helps to limit the rate of growth.
At a temperature less than 345 °C , surface reaction occurs in GeH4 , this acts as an incorporation or catalyst of Ge. McCombe et al. (2016, p.271) has asserted that into nanowire, the rate of catalyst interface is limited. Following steps need to be followed for growth.
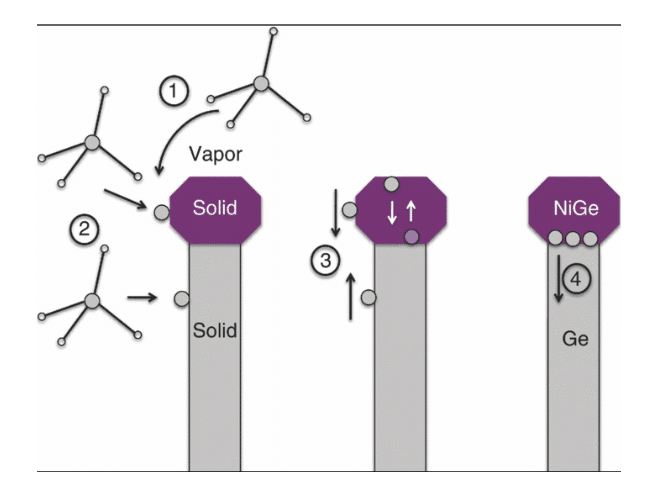
Figure 6: Catalyst interface
(Source: Dos Santos et al. 2018, p.141)
- Mass transport at gas phase in any of precursors (GeH4) into product H2 or catalyst
- Reaction of surface at presence of catalyst
- Ge diffusion across catalyst to catalyst or any nanowire interface
- Addition of some Ge atoms with catalyst or growth interface
In the VLS process, silane is applied as a metal catalyst and precursor gas. Nano clusters are heated at high temperature with Au-Si alloys. For this, liquid materials are cracked on gold surface. Incorporation of sufficient Si helps in nucleation of solid silicones.
Diameters of germanium wires depend on initial size of these droplets. Here, the condition for growth is 460°C and 40Pa. This condition reduces amorphous decomposition of silicon. For this season, VLS method is unsuitable for manufacturing polymer nanowires.
Electrospinning mechanism consists of three basic stages mentioned as below.
- Resources of high voltage
- A collector grounded by electric
- A spinneret
Different parts of this mechanism are metallic needles, polymer solution, metallic collector and power supply. This process initiates when the electric charge moves into polymer solution. Charge moves through metallic needle.
This process causes instability in polymer solution and results to induction of charges. Simultaneously, a reciprocal repulsion occurs, which produces an electric force (Dos Santos et al. 2018, p.143). This force opposes surface tension, therefore solution of polymer flows into direction of electric field.
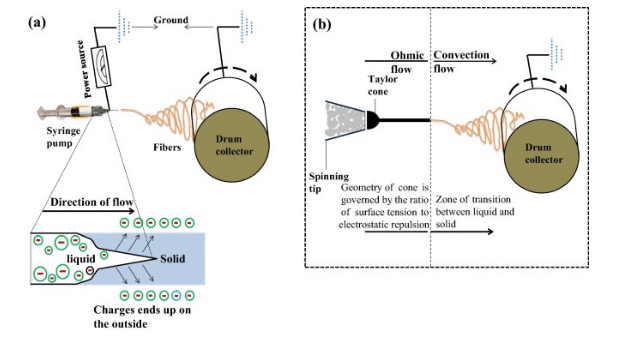
Figure 7: Solution of polymer flows
(Source: Carrera et al. 2017, p.555)
Further increment of intensity of electric field helps to deform electric droplets that assume to be a conical droplet. In this step, some ultrafine nanofibres comes out from canonical polymer droplets, these are called Taylor cone. These are collected from metallic collector and kept in optimized distance.
During production of cohesive forces, a stable jet is formed, which causes external and internal charges. This force allows whipping of liquid jet and this motion assists polymer chains.
Materials:
- PLLA
- HFP
- 6AN
- Glass cover slips
- Hermetic DSC pans
Closed environment of electrospinner lab allows controlling humidity and dryness of air. Solution is pumped out of 5 ml syringe with 22 and half gauge needle, which is attached to gamma power supply. Fibers are collected by 15*15 mm cover of glass. Through this process, solvent are removed.
Voigt model is based on uniform distribution of material’s stress. Principle assumptions of this model are mentioned as below.
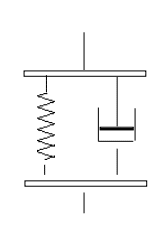
Figure 8: Voigt Model
(Source: Demers et al. 2016, p.17)
- Polymer chain has dynamic nature
- There is advanced theories including scaling concepts
- Shift factor is significant, as at a temperature, it can be imposed on shifting amount.
- Real behavior is characterized by distribution of this relaxation.
- Relaxation behavior also varies according to different length and different functions of temperature.
- Relaxation process is involved in temperature dependence
- It does not describe any stress relaxation (Kadoura et al. 2016, p.341)
- This model is characterized by a single relaxation, this helps to provide better description
- Maxwell model is not responsible for any elastic responses
The Reuss model refers to an iso-stress situation with composite perpendicular stress, named as matrix and fibers. Here, S(f) = S (m) = S (composite) Principle assumptions of Reuss Model are mentioned as below.
- Transverse strain of each of the matrix and fibres are ε (f) = S (f) / E (f)
And ε (m) = S (m) / E (m)
- Transverse strain formula for composite system is ε (composite) = Δ W (composite) / W (composite). Here, Δ W (composite) is width charge of matrix and fiber region.
- Composite stress can be viewed as series of matrix and fiber within a raw.
- Poisson ratio is ratio between resultant strain and longitudinal strain.
The upper bound of Voigt model can be defined by average of constituents.
= f1 E1 α + f2 E2 α + f3 E3 α + …….
Here, E = effective modulus of composite strain
Ei= modulus of constituent
Fi = volume of fraction of constituent
And α is a constant lies between -1 and +1
The voigt average is known as the upper bound of this model, which is E= E1 * V1 + E2 *V2
From the special case of Reuss average, it can be seen that
ER-1 = VQ EQ-1 + VF EF-1 + VC EC-1 + VQ EQ-1 + ………+ VWEW-1 + VO EO-1 + VG EG-1
And μR-1 = fQ μR-1 + fF μF-1 + fC μC-1 + ………+ fW μW-1 + fO μO-1 + fG μG-1
Carrera, D., Manganini, F., Boracchi, G. and Lanzarone, E., 2016. Defect detection in SEM images of nanofibrous materials. IEEE Transactions on Industrial Informatics, 13(2), pp.551-561. http://home.deib.polimi.it/boracchi/docs/2017_Anomaly_Detection_SEM.pdf
Demers, S., Nadeau, S. and Bouzid, A.H., 2017. Analytical evaluation of stresses and displacements of an intervertebral disc. Journal of Biomechanical Science and Engineering, 12(3), pp.16-00675. Accessed on: 16th July, 2019, Available at: https://www.jstage.jst.go.jp/article/jbse/12/3/12_16-00675/_pdf
Dos Santos, A., Viante, M.F., Pochapski, D.J., Downs, A.J. and Almeida, C.A.P., 2018. Enhanced removal of p-nitrophenol from aqueous media by montmorillonite clay modified with a cationic surfactant. Journal of hazardous materials, 355, pp.136-144. Accessed on: 16th July, 2019, Available at: https://www.researchgate.net/profile/Anthony_Downs/publication/323499386_Enhanced_removal_of_p-nitrophenol_from_aqueous_media_by_montmorillonite_clay_modified_with_a_cationic_surfactant/links/5b0975760f7e9b1ed7f7c724/Enhanced-removal-of-p-nitrophenol-from-aqueous-media-by-montmorillonite-clay-modified-with-a-cationic-surfactant.pdf
HODAEI, M. and FARHANG, K., 2015. Connection of surface roughness to hysteresis loss in spine implants. Journal of Biomechanical Science and Engineering, 10(2), pp.14-00443. Accessed on: 16th July, 2019, Available at: https://www.jstage.jst.go.jp/article/jbse/10/2/10_14-00443/_pdf
Kadoura, A., Nair, A.K.N. and Sun, S., 2016. Adsorption of carbon dioxide, methane, and their mixture by montmorillonite in the presence of water. Microporous and Mesoporous Materials, 225, pp.331-341. Accessed on: 16th July, 2019, Available at: https://repository.kaust.edu.sa/bitstream/handle/10754/593666/1-s2.0-S1387181116000202-main.pdf?sequence=1&isAllowed=y
Krutpijit, C. and Jongsomjit, B., 2016. Catalytic ethanol dehydration over different acid-activated montmorillonite clays. Journal of oleo science, 65(4), pp.347-355. Accessed on: 16th July, 2019, Available at: https://www.jstage.jst.go.jp/article/jos/65/4/65_ess15244/_pdf
McCombe, P.F. and Sears, W.R., NuVasive Inc, 2016. Vertebral disc prosthesis. U.S. Patent 9,375,322. Accessed on: 16th July, 2019, Available at: https://patentimages.storage.googleapis.com/12/79/45/bdd60877379af6/US20160038303A1.pdf
Napoletano, P., Piccoli, F. and Schettini, R., 2018. Anomaly detection in nanofibrous materials by CNN-based self-similarity. Sensors, 18(1), p.209. Accessed on: 16th July, 2019, Available at: https://www.mdpi.com/1424-8220/18/1/209/pdf
Senthamizhan, A., Balusamy, B. and Uyar, T., 2017. Electrospinning: A versatile processing technology for producing nanofibrous materials for biomedical and tissue-engineering applications. In Electrospun Materials for Tissue Engineering and Biomedical Applications (pp. 3-41). Woodhead Publishing. Accessed on: 16th July, 2019, Available at: http://repository.bilkent.edu.tr/bitstream/handle/11693/37773/bilkent-research-paper.pdf?sequence=1
Teich-McGoldrick, S.L., Greathouse, J.A., Jove-Colon, C.F. and Cygan, R.T., 2015. Swelling properties of montmorillonite and beidellite clay minerals from molecular simulation: comparison of temperature, interlayer cation, and charge location effects. The Journal of Physical Chemistry C, 119(36), pp.20880-20891. Accessed on: 16th July, 2019, Available at: https://www.osti.gov/pages/servlets/purl/1235310

