BIOT1012 Research Methods and Data Management Assignment Sample
BIOT1012 Research Methods and Data Management Assignment Sample
Summary
Gene transfer and recombinant DNA technology are playing a great role for the development of the “biopharmaceutical” industry in the last few decades. It has been noticed that the approval of genetically engineered insulin for the human has triggered the release of many other proteniousious drugs, which has led to the rapid growth of the “pharmaceutical” industry.
Thus, this study manily focuses on the role of “recombinant DNA technology” in the development of the biopharmaceutical industry. Drug development and the biopharmaceutical industry are one of the largest beneficiaries of recombinant DNA technology. It has helped in embracing the overall drug world.
Background
Biotechnology is one of the important elements that are used for the development of drugs and especially in the “pharmaceutical industry”, which can be counted as one of the biggest beneficiaries. This mainly happens because the products are so valuable, that the production process is usually not taken into account. In the views of Kaya et al. (2022), it has been noticed that the manipulation of the biological system has greatly improved the pharmaceutical industry. It has helped in the embracement of the medical world with the implementation of different biotechnology products.
This has led to the growth of two special protein drugs such as growth hormone and insulin. It has been noticed that E.coli is one of the most important elements that is used for the production of recombinant human insulin. This has led to the development of several genetically approved products.
As per the views of Szkodny and Lee (2022), recombinant technology has helped in the development of the health conditions of people by enhancing the development of biopharmaceuticals and the development of new vaccines. The recombinant technology has been effectively used in the production of several human proteins within the microorganisms.
Recombinant DNA technology involves the use of several macroscopic organisms, microorganisms, leukocytes, and the hybrid cells of tumours in order to enhance the development of pharmaceuticals. It helps in creating a safer and more effective version of conventionally produced pharmaceuticals. In reference to Welch et al. (2019), it has been noticed that previously humans suffering from diabetes used to rely on seine and bovine insulin almost similar to insulin of the human body but is not the same to and has the risk of several “zoonotic disease transfer”.
The use of “E. Coli” for the recombinant production of human insulin has helped in increasing the volumes and the efficiency of the production of biopharmaceuticals (Modak et al. 2022). It has a significant reduction of cost, making the products that contain protein available to the public. This process involved subsequent expression and cloning of the “hetero-dimeric” human insulin gene in E. coli where the biosynthesis is translated to a complete human protein in a different non-human biological system.
The efficiency of protein drugs has been brought by the development of “recombinant DNA technology” and has made the whole process easier to access of essential drugs to the general public. However, it has been noticed that the greatest benefit was manily derived from the giants of the transitional pharmaceutical (Afshari-Mofrad and Salim, 2020). The research that has been funded in these areas has lead to the development of essential drugs.
Thus, with the approval of these “genetically engineered therapeutics,” there are several genetics molecular companies that came into being. The implementation of the recombinant technology has helped in the production of several growth hormones an important peptide drug, making it easily available in the market. it has been noticed that before the implementation of the “recombinant DNA technology,” the growth hormone was manily derived form the cadavers, leading to the rise of several “Creutzfeldt- Jakob diseases”.
Work Plan & Project Objectives
This project is manily based on the implementation of “recombinant DNA technology” in the development of the biopharmaceutical industry. The main objective for the implementation of this technology is to develop several growth hormones and is manily used for the production of vaccines and several protein therapies (Nash, 2022). This technology is used for several clotting factors, and also for treating hemophilia and for the development of gene therapy.
It is one of the important research tools that is used in biology, it is allowing scientists to manipulate the fragments of DNA for the purpose of studying them in the lab. This process involves a variety of laboratory methods to put the piece of DNA in yeast or bacterial cell.
It has been noticed that the appropriate DNA should be taken into account and needed to be developed in a proper manner in order to implement it in the biopharmaceutical industry (Kandemir et al. 2020). As recombinant technology is inserted in the host organism for the production of the new DNA within the body it should be done within a limited period of time, a minimum of one week. The whole process of the study will require one year or less to get completed.
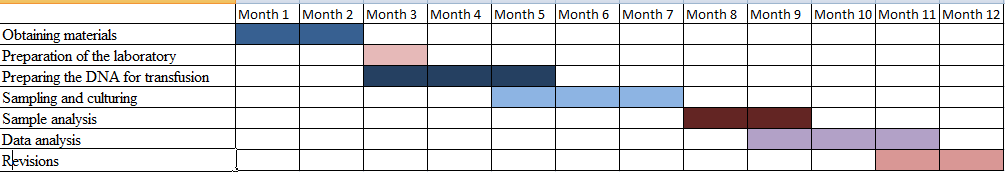 Figure 1: An estimated timeline for the completion of the project (Source: Ms- Excel)
Figure 1: An estimated timeline for the completion of the project (Source: Ms- Excel)
It has been noticed that once the DNA is transfused in the body, the media will seem to be sampled for at least 95 hours. It will help in the development of the hormone that is essential for the body. It is quantified with the help of “Western blot analysis”. The data that has been collected will be analyzed in a statistical manner, in order to determine the difference and to see if it is significant.
Benefits And Impact
The recombinant technology is playing a great role in the development of the health condition of the people, with the development of new vaccines and the decelopment of several drugs. In the views of Uppal et al. (2020), it is helping in the implementation of several unique impacts for bringing great change in the life of the human .
This technology has several disciplinary applications and the potential in order to deal with greater propsects of life such as improving health, resistance to adverse environments, enhancing food sources, and several others. The recombinant technology, modification of gene, and gene therapy have been widely used for treating several serious diseases and for the purpose of bioremediation. In reference of Benyahia et al. (2020), due to the tremendous range of applications and the advancement in the field of recombinant DNA technology, there is a huge growth in the drug industry of the world.
It has helped in increasing the importance of the production of vaccines and several protein therapies such as the production of human insulin, and the development of growth hormone. As per the views of Arias et al. (2022), the most common advantages that have been seen with the implementation of this technology is high-mass production, lack of human pathogens, low cost, and several other.
The main benefits of the implementation of recombinant technology in the development of the biopharmaceutical industry as it helped in increasing the efficiency of the medicines and manufacturing the effective version of the conventionally produced pharmaceuticals. It has helped in increasing the safety and the health of patients through the fact-monitoring process. As per the journal of Chirmule et al. (2021), it helps in enhancing the manufacturing process of antibodies and proteins with a defined uniformity and specificity. It is manily used for the development of patients with any kind of malfunctions in the pituitary gland.
The application of the “recombinant technology of DNA” has helped in evolving the overall condition of the biopharmaceutical industry. In the views of Uppal et al. (2021), it is allowing scientists to manipulate the DNA fragments for studying them in the lab. The application of this technology is helping in the “mutation” process.
It is also allowing people to fight different chronic diseases such as cancer, diabetes, and several others, with the help in the development of several vaccines in the pharmaceutical market. As recombinant products are generally getting manufactured in the laboratory, it is one of the safer options than any kind of plasma-derived products, as they avoid any kind of potential blood-borne disease. It is alos helps in reducing the transmission of any blood-borne disease.
The presence of the host organism is one of the important factors for the development of the recombinant technology of DNA. In the views of Paradia et al. (2023), it had made the whole process of the vaccines very easier, making it cost-effective in nature. It is allowing people to get access to health benifits enhancing the condition of health of people.
As the chance of contamination is alos less in this process the rate of getting any side effects is alos less in this process. It is alos helps in increasing the immune system of the body as taking genes from the strong body helps in increasing the capability of the body. It is helping the pharmaceutical industry to produce vaccines on a large scale, as this process normally includes yeast in order to produce antigens. It is helping in the production of large-scale vaccines in an effective cost manner and increasing the efficiency of the industry.
Costing
The presence of the funds is one of the necessary factors for the development of the funds. A senior researcher was involved in the project for 1 year to see the aspect of the study at 0.7 FTE. An assistant researcher was alos hired at 0.6 FTE.
| Name of variables | Volume of Expenses | £ |
| Personnel | Expenses | |
| Research Assistant | 0.6 FTE | 10,000 |
| Senior researcher | 0.7 FTE | 20,000 |
| Materials and equipment | ||
| vectors, selection kits | 4000 | |
| Research organisms | 1,250 | |
| Consumables | 10,000 | |
| Total Estimated cost | £45,250 |
Figure 2 – A breakdown of estimated costs (Source: Ms- excel)
Reference list
Benyahia, B., Brumano, L.P., Pessoa, A. and da Silva, F.V.S., 2020. Biopharmaceutical development, production, and quality. In New and Future Developments in Microbial Biotechnology and Bioengineering (pp. 69-89). Elsevier
Chirmule, N., Bhat, S. and Mondal, S., 2021. Biopharmaceutical Development in India: Recommendations on Collaboration and Innovation to Enable Affordable Healthcare. Drug Discovery and Drug Development: The Indian Narrative, pp.255-281.
Kandemir, B., Maranho, J. and Kondo, W., 2020. A Systematic Review of Synthetic Biology-A New Era in Biopharmaceutical Drug Development. Biomedical Journal of Scientific & Technical Research, 29(1), pp.22171-22176.
Kaya, S.I., Cetinkaya, A., Caglayan, M.G. and Ozkan, S.A., 2022. Recent biopharmaceutical applications of capillary electrophoresis methods on recombinant DNA technology‐based products. Electrophoresis, 43(9-10), pp.1035-1049.
Modak, M., Nyayanit, N., Sivaram, A. and Patil, N., 2022. The Recombinant DNA Technology Era. In A Complete Guide to Gene Cloning: From Basic to Advanced (pp. 1-14). Cham: Springer International Publishing.
Nag, N., Khan, H. and Tripathi, T., 2022. Strategies to improve the expression and solubility of recombinant proteins in E. coli. In Advances in Protein Molecular and Structural Biology Methods (pp. 1-12). Academic Press.
Nash, M.A., 2022. Elastin-like polypeptides: protein-based polymers for biopharmaceutical development: Medicinal Chemistry and Chemical Biology Highlights. Chimia, 76(5), pp.478-478.
Paradia, P.K., Bhavale, R., Agnihotri, T. and Jain, A., 2023. A Review on Edible Vaccines and Biopharmaceutical Products from Plants. Current Pharmaceutical Biotechnology, 24(4), pp.495-509.
Szkodny, A.C. and Lee, K.H., 2022. Biopharmaceutical manufacturing: Historical perspectives and future directions. Annual Review of Chemical and Biomolecular Engineering, 13, pp.141-165.
Uppal, A., Chakrabarti, R., Chirmule, N., Rathore, A. and Atouf, F., 2021. Biopharmaceutical industry capability building in India: report from a symposium. Journal of Pharmaceutical Innovation, pp.1-8.
Uppal, A., Koduri, C.K., Yadlapalli, S., Chirmule, N., Chakrabarti, R. and Atouf, F., 2020. Recommendations for enhancing quality and capability of Indian biopharmaceutical industry: summary of a workshop. Journal of Pharmaceutical Sciences, 109(10), pp.2958-2961.
Welch, J.T. and Arden, N.S., 2019. Considering “clonality”: A regulatory perspective on the importance of the clonal derivation of mammalian cell banks in biopharmaceutical development. Biologicals, 62, pp.16-21.
Know more about UniqueSubmission’s other writing services:


Thanks, I have just been looking for information about this subject for a long time and yours is the best I’ve discovered till now. However, what in regards to the bottom line? Are you certain in regards to the supply?