Energy levels for quantum numbers Sample
Question 1: Discuss the energy levels for quantum numbers 1-3
Answer :
Quantum Numbers
“The wave equations used to describe the electron configuration require at least 3 variables called quantum numbers. Changing the quantum numbers, the variables in the wave equation, changes the energy of the electron. Each electron has its own unique set of quantum numbers. The shapes shown for the quantum orbital are created by graphing the wave equations in three dimensions. The outer boundary of the shape is the cut-off point for the location of the electron 90% of the time. Since the electron in an orbital travel randomly both in direction and speed, pinpointing its location or path is impossible. Thus an orbital represents the container where an electron is probably located, and each orbital is a graph of the wave equation for a specific energy.”
The First Quantum Number: Energy level or shell, n
“The first quantum number describes the radius the electron exists away from the nucleus. So n = 1 means that the electrons with this quantum number will be closest to the nucleus. n = 2 will be in the second layer, n = 3 will be in the third layer, and so on up to n = 7.”
The Second Quantum Number: Orbital Shape subshell, ℓ
“The second quantum number determines the shape of the orbital, which is the location where electrons can be found in the atom. The number tells you how many nodes are in the shape, where a node is the crossing point in the shape. So ℓ = 0 has no crossing points and will be just a sphere like we expect of an atom (recall the electron location, or electron cloud when all orbitals are combined, is the boundary of the atom). ℓ = 1 has 1 crossing point and looks like a figure-8. ℓ = 2 has 2 crossing points and looks like a four-leaf clover. ℓ = 3 has 3 nodes and is a bit complicated ( ℓ = 4 is the highest orbital for this number). The shapes are labeled by either numbers or letters: ℓ = 0 = s; ℓ = 1 = p; ℓ = 2 = d; ℓ = 3 = f.”
The Third Quantum Number: Orientation in Three Dimensional Space
“The third quantum number is used to designate orientation in space. The figure-8 shape with ℓ = 1, has three shapes needed to completely fill the spherical shape of an electron cloud. That is combining figure-8 shapes going up and down (z-axis), left and right (x-axis), and back and forth (y-axis), the entire electron cloud will be filled with figure-8 orbitals. It takes three orientations for figure-8 orbital, ℓ = 1, five for the cloverleaf orbital, ℓ = 2, and seven for ℓ = 3 ( ℓ + 1, or consecutive odd numbers).”
Question 2: Describe the shapes of s,p and d orbitals. Diagram may be used
Answer: “There are four different kinds of orbitals, denoted s, p, d and f each with a different shape. Of the four, s and p orbitals are considered because these orbitals are the most common in organic and biological chemistry. An s-orbital is spherical with the nucleus at its centre, a p-orbitals is dumbbell-shaped and four of the five d orbitals are cloverleaf shaped. The fifth d orbital is shaped like an elongated dumbbell with a doughnut around its middle.”
The Shape of s Orbitals
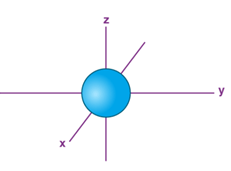
“The boundary surface diagram for the s orbital looks like a sphere having the nucleus as its centre which in two dimensions can be seen as a circle. Hence, we can say that s-orbitals are spherically symmetric having the probability of finding the electron at a given distance equal in all the directions.”
The Shape of p Orbitals
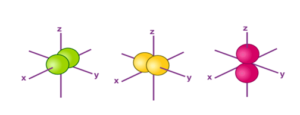
“Each p orbital consists of two sections better known as lobes which lie on either side of the plane passing through the nucleus. The three p orbitals differ in the way the lobes are oriented whereas they are identical in terms of size shape and energy.”
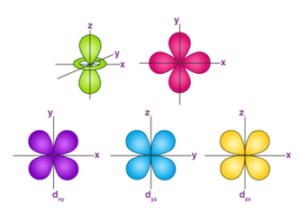
The Shape of d Orbitals
“The magnetic orbital quantum number for d orbitals is given as (-2,-1,0, 1,2). Hence, we can say that there are five d-orbitals.These orbitals are designated as dxy, dyz, dxz, dx2–y 2 and dz2.”
Q3: Explain the distribution of different particles within the atomic structure of elements and explain their relative masses and charges
Ans : “All matter including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: protons, neutrons, and electrons. As summarized in Table, protons are positively charged, neutrons are uncharged and electrons are negatively charged. The negative charge of one electron balances the positive charge of one proton. Both protons and neutrons have a mass of 1, while electrons have almost no mass.”
Table: Charges and masses of the particles within atoms
Proton: “A proton is a subatomic particle, symbol p or p⁺ with a positive electric charge of +1e elementary charge and a mass slightly less than that of a neutron.”
Neutron: “Neutron is a subatomic particle, symbol n or n⁰, which has a neutral charge, and a mass slightly greater than that of a proton”
Electron: “The electron is a subatomic particle, symbol e⁻ or β⁻, whose electric charge is negative one elementary charge.”
“The element hydrogen has the simplest atoms, each with just one proton and one electron. The proton forms the nucleus, while the electron orbits around it. The positively charged protons tend to repel each other, and the neutrons help to hold the nucleus together.”
Q4: a) Draw an energy level diagram of scandium(sc) in terms of s, p and d sub-levels
Ans:
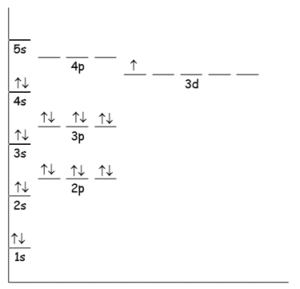
Electronic configuration: 1s22s22p63s23p64s23d1
- b) Finish the following electron configurations
- i) Sc
Ans: 1s22s22p63s23p64s23d1
- ii) Sc3+
Ans: 1s22s22p63s23p6
Q5. Define an isotope and explain how the existence of isotopes results in relative atomic masses (RAM) of some elements not being whole numbers
Ans: “Isotopes can be defined as the variants of chemical elements that possess the same number of protons and electrons, but a different number of neutrons. In other words, isotopes are variants of elements that differ in their nucleon numbers due to a difference in the total number of neutrons in their respective nuclei. For example, carbon-14, carbon-13, and carbon-12 are all isotopes of carbon.Carbon-14 contains a total of 8 neutrons, carbon-13 contains a total of 7 neutrons, and carbon-12 contains a total of 6 neutrons.”
“The atomic mass is not a whole number even though the masses of protons and neutrons are whole numbers is because of the existence of the isotopes of the atoms of the same element. For example, the element hydrogen has three isotopes and they are hydogen-1 with a mass of 1, hydrogen-2 with a mass of 2 and hydrogen-3 with a mass of 3. The most abundant of these isotopes is hydrogen-1 and the other two isotopes are rarely found.The tabulated atomic mass is the over-all average mass of all the isotopes. Averaging 1, 2,and 3 will give a decimal value when their percentages of existence are being considered.”
Q6: The table below gives the relative abundance of each isotope in a mass spectrum of a sample of oxygen
| m/z | 16 | 17 | 18 |
| Relative Abundance(%) | 99.762 | 0.038 | 0.200 |
Use the data above to calculate the relative atomic mass of this sample of oxygen.
Give your answer to 3 decimal places
Ans:
| m/z | 16 | 17 | 18 |
| Relative Abundance(%) | 99.762 | 0.038 | 0.200 |
| relative atomic mass | 15.962 | 0.006 | 0.036 |
Q7. Using periodic table explain what information is provided by mass number and atomic number. Give at least two examples to demonstrate your understanding
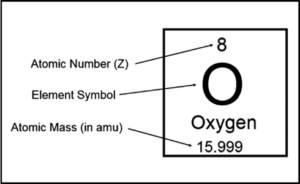
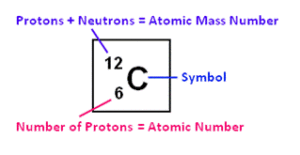
“Atomic mass number is the total number of protons and neutrons. Atomic number is equal to the number of protons.”
Q8. Describe an ion
“An ion is a particle, atom or molecule with a net electrical charge. The charge of the electron is considered negative by convention. The negative charge of an ion is equal and opposite to charged proton considered positive by convention.”

