Great Food Science and Innovation Assessment Sample
Introduction ( Food Science and Innovation Assessment Sample)
This research study emphasizes on the factors affecting adsorption on various oil phases in oil and water emulsions. In order to get a clear understanding on the research work, factors which impacts the adsorption of emulsifier into the oil phase need to be studied.
As stated by Du, et al., (2010), adsorption is illustrated as the adhesion process through which the gases or liquid particles develop a molecular or atomic adsorbate film on the liquid or solid surface.
In the process of adsorption, the particles of solid or liquid is only concentrated on the top layer and do not able to penetrate through the layer of adsorbent.
On the other hand, another key term associated with the research work is emulsion which can be defined as a kind of colloid which are generally formed due to the mixture of two immiscible liquids, as explained by McClements, (2010).http://Food Science and Innovation Assessment Sample
Gum Arabica has been considered as the emulsifier in this research work and it comprises of polysaccharides and glycoproteins which mainly compose of galactose and arabinose.
This gum is extracted from the acacia tress founded in provinces of sub – Saharan Africa such as Sudan and Senegal.
The literature review has been developed based on the aims and objectives of this study. This study puts emphasis on Gum Arabic as the commonly used emulsifier, therefore the characteristics of emulsifier needs to be studied.
But prior to that, basic understanding of emulsion and the applications of emulsifier are also being discussed in this research work. Characteristics of Acacia family is also concentrated within this research work.
The factors which impact the adsorption of oil phases in oil and water emulsion such as such as temperature, molecular weight, surface tension, hydrophobicity, viscosity and pH are also elaborated in this research study.
Conceptual framework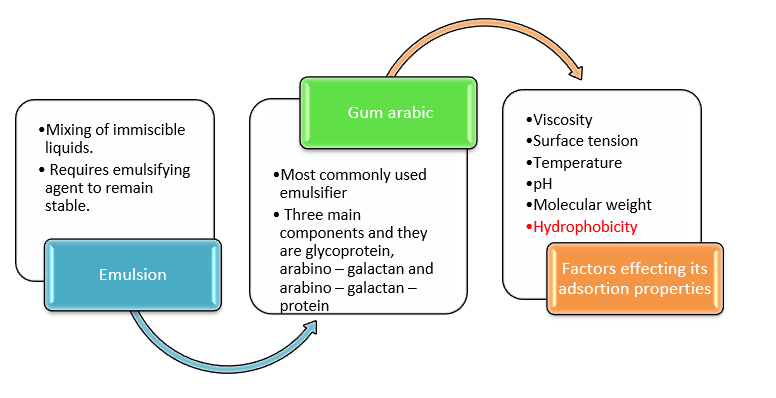
Source: Author
This section of the literature review highlights the key components of this research study. The three key components are the emulsion process, emulsifiers i.e.
gum Arabica and the factors such as viscosity, surface tension, pH, molecular weight, hydrophobicity and temperature which would impact its adsorption in oil and water emulsion.
As an emulsifier, gum Arabica has the role of stabilizing the emulsion process but the aforementioned factors influences its characteristics in the adsorption process.
Thus, concept emulsion, characteristics of gum Arabica and the stated factors are the most important elements of this literature review.
Concepts of emulsions
Emulsion is often seen as a mixture of two liquids that are immiscible in characteristics. The prime example emulsion are milk, mayonnaise and butter.
According to Tedros, (2013), the process of converting a mixture of liquids into an emulsion is known as emulsification. Properties of emulsion suggests that it appears to be white or cloudy when formed due to the fact that the light is being scattered off from the interface layer of two liquids.
Therefore, concentration of the emulsion explains its color. The emulsion even appears as yellow as the distorted color would lead to longer wavelength when the emulsion is highly concentrated.
The emulsion also appears to be translucent in cases of microemulsions and nano emulsions. These emulsions lack static internal structure because of their liquid state.
In a liquid matrix, droplets are being distributed well enough and different kind of emulsions can be observed in two liquids. For example – water and oil emulsion can create either water in oil emulsion or oil in water emulsion and there are even chances of multiple emulsions like water in oil in water.
According to Charoen, et al., (2011), majority of the emulsion remain unstable until they are treated with emulsifiers. These stable emulsions can be turned into unstable emulsion by inactivating the emulsifying agent.
Stability of an emulsion is defined as the capability of emulsion to maintain its properties over a time period but normally four types of instabilities can be seen in emulsion and they are coalescence, flocculation, Ostwald ripening and creaming.
Flocculation occurs when the particles of the dispersed phase stay together due to the attractive forces and it leads to unstable emulsion. Coalescence is another form of instability in emulsion which occurs due to the creation of larger droplets from discontinuous phase and it eventually lead to higher average particle size with time.
Ostwald ripening is described as the alteration of inhomogeneous structure within a time period. Creaming happens due to the centripetal force or buoyancy in a mixture of two liquid which results in droplets go to the highest level of emulsion.
The attention has shifted towards microemulsions and nano emulsions which are generally thermodynamically stable over traditional macroemulsions due to their stability.
Since microemulsions have minute droplet sizes which develop them to be transparent instead of opaque.
From the article by Anton & Vendome, (2011), generally macroemulsions need vigorous mixing but in case of microemulsions or nano emulsions, no high – intensity mixing is required due to their thermodynamically stable characteristics.
But microemulsions need more emulsifiers as comparison to macroemulsions.
Emulsifiers are used in emulsion by developing a physical barrier which do not allow droplet to coalesce. The selection of an emulsifier is highly crucial for the emulsification process.
Based on the article by Gadhvi, (2014), in order to do so, HLB (hydrophilic-lipophilic balance) needs to be calculated or a mixture of emulsifiers can be used. Normally the HLB scale ranges from 0 to 20 for emulsifiers.
Emulsifiers with value more than 10 in HLB scale have inclined towards being more hydrophilic and therefore, more effective in stabilizing oil in water emulsions.
Similarly, emulsifiers with less than 10 in HLB scale are termed as more hydrophobic and therefore, more effective in stabilizing water in oil emulsions. Moreover, different liquids have their different HLB specifications, for example – vegetable oil emulsion requires 7 – 8 HLB rating emulsifier.
The application of emulsifiers are seen in many sectors and purposes like production of plastics and synthetic rubber, manufacturing of cosmetics, food and beverage sectors etc.
Application of emulsifier
According to Charoen, et al., (2011), emulsifiers are described as the surface – active substances or additives which are basically used to mix two liquids.
Emulsifiers are usually described as surfactants which can be further categorized as hydrophilic and lipophilic. Normally, the emulsion process takes place between the greasy and aqueous parts, they form their different layers and segregate from each other.
But with the help of emulsifiers, a stable emulsion can be achieved. An emulsifier has lipophilic tail which is directed towards the greasy phase and hydrophilic head which is directed towards aqueous phase.
These interfaces mitigate the surface tension of the droplets of oil and thus can be breakdown into smaller particles which facilitates in mixing and thus eventually results in stable emulsion.
Being a surface active agents, they are able to decrease the surface tension of the aqueous phase and remain in the interface between oily and aqueous phase thus developing a connection between these two phases and create an emulsion.
The fact that it circumvents the oil and water mixture from coalescing by developing barriers around the droplets helps to create stable emulsion. Along with the application of making stable emulsion, these emulsifiers have the capabilities to react with food ingredients.
As stated by Dons, et al., (2011), food products such as chocolate, breads, margarine, ice – cream etc. are made with the help of emulsifiers. Emulsifiers can be added as an additive or can be naturally present in the foods.
For example – in stable emulsion of mayonnaise, lecithin is used as an emulsifier to stabilize the lemon juice, oil and egg mixture. They are also used to enhance the quality of the food such as in salad dressing.
It also helps in increasing the shelf life of the food because shelf life of food is directly proportional to the quality of the food. Even they are widely used in skin care and cosmetic products and they are termed as ten sides when preferred for skin cleaning uses.
The other applications of the emulsifiers are they often act as an aerating agent, crystallization inhibitor and even starch complexing agent.
Characteristics of Acacia family
Acacia is a genus of more than 1200 species of shrubs and trees of the family of pea. These shrubs and trees are found in subtropical and tropical regions across the globe, especially in Africa, Asia, America and Australia.
These species normally show the properties of savanna vegetation. Despite being well diverse, they show similar characteristics.
Based on the article by Muhammad, et al., (2016), the distinctive leaves of acacia takes a peculiar shape of finely divided leaflet which account for its fernlike appearance. Leaflets can be seen absent altogether in many of the Pacific and Australian species of acacia.
As explained by Livraison & Vandeveld, (2017), this fragrant small flowers of acacia trees give them a distinctive look as they are arranged in cylindrical clusters or compact globular structure.
The fuzzy appearance of the flowers of the acacia is due to the yellow color of the flower and apiece stamens. It develops into pea – lookalike flower which consists of five petals and the color of the flower varies from white or yellow and even purple or red cultured.
Based on the species of acacia, the appearance of the fruits change which are normally considered as legumes. Different acacia species have found their economical uses, as suggested by Shi, et al., (2019).http://Food Science and Innovation Assessment Sample
For example – Acacia Senegal which are found out in sub – Saharan region of Africa provides gum Arabica. It has its wide array of uses in different sectors, ranging from food sector to pyro techniques.
The fact that tannin is found in high quantity in the barks of most species of acacia, therefore it has also used for dyeing and tanning purposes and even in pharmaceuticals.
Some of the Australian species of acacia such as silver wattle, golden wattle and green wattle are sources of tannin and even some of the species of acacia provides high quality timber such as yarran, Australian blackwood etc.
On the other hand, other members of acacia family are considered valuable for essential oils, perfume and lac.
Acacia normally grows in sunny and dry weather conditions and eventually grows to at least a height of 40 ft.
There are even acacia trees which can grow to 70 ft height and a diameter of 3 ft. The leaves of the acacia trees are seen as pinnate and green leaves. Even some of the non – Australian acacia species have thorns which protect them from the herbivores.
It can also offer many benefits to the ecosystem, but the fact that many species of acacia are already branching out to new ecosystems and it is because of their nature of growth, as explained by Noria & Shackleton, (2019).http://Food Science and Innovation Assessment Sample
Reason such as economic value and attractive landscaping have allowed these species to enter into the new ecosystems. The different species of acacia are used for different purposes.
They are being used as a healing agent in tropical treatments. Even the powdered acacia has its application in promoting oral care and found as a component in herbal toothpaste.
Kira, et al., (2018) have suggested that the presence of dietary fibers which are water – soluble in acacia gum has helped it to be a good source of fiber and helps in managing the cholesterol level of human beings.
Another example of health benefit of acacia gum can be seen in mitigating the body fat. Acacia gum can also provide relief to patients suffering from inflammation and irritation.
Another element of acacia family, Acacia grigio plant, is used to restrict the loss of blood from cuts and wounds and often characterized as a better wound healing than the options found in the market.
Characteristics of Gum Arabica and it’s applications
According to Nau, et al., (2016), gum Arabica is normally extracts from the branches and stems of the acacia trees in the form of sticky liquid.
There are in excess of 1000 species of acacia but for the manufacturing of gum Arabic acacia trees found across Sahelian belt of Africa, most precisely Acacia Seal and Acacia Senegal are considered.
They are often considered as natural gum which comprises of hardened sap of the different acacia species. One of the key characteristics of gum Arabica is its composition.
It is a mixture of polysaccharides and glycoproteins which mainly compose of galactose and arabinose. Gum Arabica is odorless and does not impact the color or odor of the mixture in which it would be used.
From the article by Fang, et al., (2010), with the application of hydrophobic interaction chromatography, proteins and polysaccharides present in gum Arabic can be segregated into three main parts and they are glycoprotein, arabino – galectin and arabino – galectin – protein.
The amount of these components depend on the age of the tree, soil, geography etc. Arabino – galectin – protein or AGP is the gum main’s active component which plays the most critical role in the emulsifying activity.
It has been seen that protein rich components adsorb more from the surface of the droplets and through UV absorbance and RI clearly shows after and before gum Arabic’s GPC elution profile while developing limonene oil-in-water emulsion.
UV absorbance conducted at 280 nm is responsive to proteinaceous material eluting concentration. On the other hand, refractive index is responsive to gum Arabica eluting concentration.
Difference in the two UV absorbance and two RI profiles before and after emulsification explains the quantity of protein and gum Arabica adsorbed respectively, as stated by Al-Assaf, et al., (2009).http://Food Science and Innovation Assessment Sample
It also illustrates the fact that the adsorbed molecules has protein and molecular mass dependency is not present. Emulsification efficiency of gum Arabic is lost when it is treated with proteolytic enzyme so that protein can be removed.
According to Al-Assaf, et al., (2009), in order to create an emulsion, the concentration of gum Arabica required would be higher than what is need for creation of pure protein as the proteinaceous component of gum Arabica is only used in emulsion.
For example – concentration of approximately 12 % of gum Arabica is required for the production of orange oil emulsion of 20 % and the emulsion formed is quite stable, even for longer time frames with no sign of coalescence happening.
Emulsification property of the gum Arabica solutions can be influenced by the prolong heating of the gum which leads to the precipitation of proteinaceous component from the solution.
According to Kennedy, et al., (2012), new generation of gum Arabica has been produced through new process which basically enhances the emulsification properties and found its place in commercial uses.
This new generation of gum Arabica is known as Supergun and it basically contains all the properties of traditional gum but with enhance functionalities.
This Supergun is molecularly and chemically similar to the original gum but the improvement of functional and physical performance is due to difference in spreading of proteinaceous components.
The maturation process of this gum surges the intensity of arabino – galectin – protein with time.
The other characteristics of gum Arabica is it is edible and soluble in water and thus can be used as an emulsion stabilizer in food industry, as explained by Liu, et al., (2010).http://Food Science and Innovation Assessment Sample But it remains insoluble in oil in other organic solvents.
When dissolved in water, gum Arabic provides a clear solutions whereas the color of the solution might change from very pale yellow to orange brown and the pH is approximately 4.5.
Acacia Senegal has a very balanced structure which accounts for its small hydrodynamic volume and compact molecules and this leads to being viscous only at high concentrations, as indicated by Castellani, et al., (2010).http://Food Science and Innovation Assessment Sample
It has been recently found out that gum Arabic also has shear thinning properties at low shear rates and when the gum Arabica solutions is provided with a constant shear, viscosity of it surges with time and eventually form weak gel characteristics when kept for more than 2 hours.
At this point of time, there is a significant increase in the storage modulus of gum Arabica solutions.
The viscosity of the gum solutions falls down when come in contact with the electrolytes because of the charge screening and undissociated nature of carboxyl group is observed at low ph. This fact can be justified on the basis of polyelectrolyte nature of gum Arabic.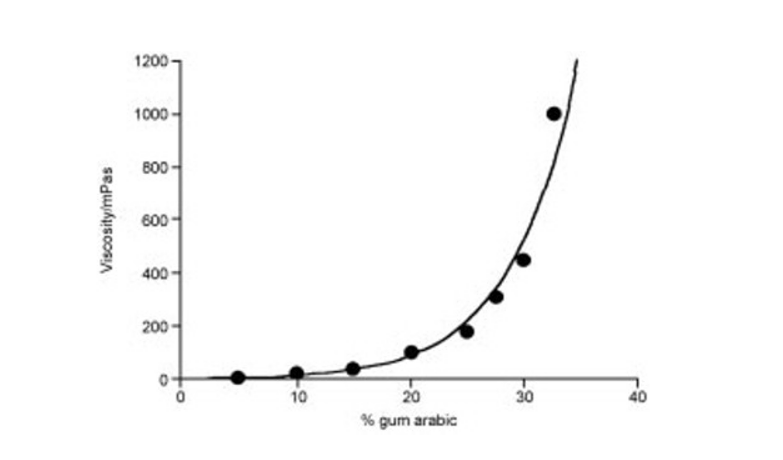
Figure 1: Viscosity of gum Arabica based on concentration as a function, Source: (Al-Assaf, et al., 2009)
Based on the article by Mujawamariya, et al., (2012), food industry prefers gum Arabica as its significant emulsifier, thickening agent and stabilizer for products such as chewing gum, soft candy, fillings etc.
Due to its property of viscosity increasing agent and emulsifying agent, it has also found its uses in cosmetics and pharmaceutical sectors. The fact that it acts as a traditional binder also outlines its use in painting and photography because it is highly soluble in water.
It has been also used as wine fining agent by the wine makers. The beverage industry also takes a keen interest on gum Arabica as an emulsifier due to its stabilizing nature and acts as a binder for flavoring and sweeteners in soft beverages.
It has been seen using as gnome syrup (mixture of gum Arabica and sugar in water) in cocktails to circumvent crystallization of sugar and thus retains a smooth texture. Due to its non – toxic nature, it can be consumed by human but it remains indigestible to both animals and human beings.
They are also being used in ceramics as an additive to glaze the ceramics. Basically, it performs the role of a binder which adheres glaze and clay together prior being fired.
It also performs the role of a deflocculant and thereby improves the fluidity of the mixture. Another use of gum Arabica can be seen in photography where cultured photographic emulsion is being created when potassium dichromate or ammonium is mixed with gum Arabica, as suggested by Casentino, (2015).http://Food Science and Innovation Assessment Sample
It also performs the role of water solution binder in pyro techniques. In addition to all these applications, it is also used in therapeutic actions and medical purposes like antidiabetic, antioxidant, hypoglycemic, antiulcer and immunomodulatory.
Even the local communities use gum Arabica to address health issues such as cardiac, renal and hepatic complications. It has even showed its uses in losing weight, taking control of obesity and maintaining cholesterol level. It is also used in the form of mucilage in adhesives for paper, metal and glass products.
Elaboration of factors such as temperature, molecular weight, viscosity, hydrophobicity, surface tension and pH of gum Arabica which impact the adsorption into the oil phase
From the article by Dickhut, et al., (2017), temperature plays a huge role in maintaining emulsion stability as it impacts the physical properties of aqueous and oily phases, solubilities of emulsifiers in water and oil phase and interfaces.
The major impact of the temperature is seen on how it regulates the viscosity of the emulsions as increase in the temperature leads to the decrease in viscosity.
Based on the article by Zhang, et al., (2016), temperature surges the thermal energy within the droplets and hence also surges the drop collisions frequency.
Increase in temperature also mitigates the interfacial viscosity which eventually leads to faster drop coalescence and rate of film – drainage.
According to Darkish, et al., (2018), slow destabilization of the water and oil emulsion interface is observed with the surge of temperature. Still, kinetic barrier in context to drop coalescence is observed at higher temperatures.
Build – up rate of interfacial films of the emulsion is also influenced by the temperature and this build – up influences is carried out by altering the interface characteristics and adsorption rate.
Temperature also impacts the compressibility of the film by altering the solubility of oil emulsifier in the bulk phase. At higher temperature, aging and slow degassing are also seen which leads to change in the behavior of the interface of the emulsion.
As indicated by Castellani, et al., (2010), emulsification property of the gum Arabica solutions is impacted by increase in temperature of the gum which leads to the precipitation of proteinaceous component from the solution.
According to Davidov-Pardo, et al., (2016), pH has a major influence on the stability of the emulsion.
The addition of bases and inorganic acids in the emulsion leads to ionization of interface which alters the characteristics of the film. In fact, the pH of water also impacts the interface’s rigidity.
The interfacial layer created by asphaltenes is considered the strongest in acid and with the increase in pH, it becomes weaker. On the other hand if the medium is alkaline, the interfacial layer becomes weak and even can be seen into mobile films. It also impacts the kind of the emulsion formulated.
From the article by Zees, et al., (2015), water in oil emulsions are produced by low pH solutions i.e. acids whereas oil in water are produced by high pH solutions i.e. bases.
The viscosity of the gum solutions falls down when come in contact with the electrolytes because of the charge screening and undissociated nature of carboxyl group is observed at low ph. This fact can be justified on the basis of polyelectrolyte nature of gum Arabic.
According to Kasia, (2018), molecular weight is defined as the total atomic weight of the atoms present in a molecule and even described as the one mole mass of the product. Gum Arabica has a 106 Da molecular mass which are extracted from the bark or sac of acacia tress.
As suggested by Kennedy, et al., (2012), the molecular mass of gum Arabica extracted from the trees in Sudan has shown a potential increase from 250,000 when they are five years old and 450,000 when they are fifteen years old.
Even the molecular mass of arabino – galectin – protein also surges with the growth of tree.
This increase in molecular mass is done by a new process which allows the elevation of the maturation process of gum Arabica. The molecular structure of the gum Arabica is highly branched and thus explaining its solubility characteristics.
The impact of molecular weight of gum Arabica in oil and water emulsion is observed at neural pH and the effect is seen at the distributions of droplet sizes.
Based on the article by Cui, et al., (2013), the high molecular weight fraction which is protein rich allows the gum Arabica to be adsorbed into oily phase surface whereas the main chain of polysaccharide hinders coalescence through repulsion forces.
The higher molecular size of gum Arabica makes it slower to be adsorbed.
As stated by Cathleen, (2015), viscosity is often explained as the resistance between the layers of the fluid while changing their shape. It is also described as the opposite of fluidity.
According to Kennedy, et al., (2012), compact molecules of the gum Arabica is due to its highly balanced structure derived from Acacia Senegal and it also develops a small hydrodynamic volume.
This has resulted in gum Arabica becoming viscous only when subjected to higher temperatures.
With comparison to the viscosity of common thickening agent such as sodium carboxymethylcellulose and xanthan gum, it has been seen that at lower shear rates, 1% of sodium carboxymethylcellulose and xanthan gum has a higher viscosity than 30 % of the gum solution.
As explained by Kennedy, et al., (2012), viscosity of gum Arabica is independent of shear rate and follows Newtonian behavior with respect to its viscosity and on the other hand, sodium carboxymethylcellulose and xanthan gum reflects non – Newtonian characteristics.
In a contradictory report, it has also been observed that gum Arabic’s viscosity surges with time when influenced under constant shear and it leads to lesser gel characteristics.
The polyelectrolyte nature of the gum Arabica decreases its viscosity when reacted with electrolytes. Generally, it shows lower viscosity when put into cold water.
Based on the article by Biko, et al., (2018), surface tension is defined as the intention of the fluid surface to reduce into the minimum possible area and it occurs due to the cohesive forces between the molecules of the fluid.
Gum Arabic’s dynamic surface tension has been highlighted in the below figure at different concentrations. Based on the article by Cao, et al., (2013), surface tensions drops down with the increase in time and normally takes the structure of a plateau.
Even after 1000s, adsorption equilibrium is hard to achieve by at all concentrations. The adsorption process of gum Arabic is slower due to its higher molecular size.
As stated by Cao, et al., (2013), it has been observed that surface tension equilibrium for 3 % wt. of gum Arabica is 55 MN m-1 and this signifies the fact that there is lower surface activity in gum Arabica.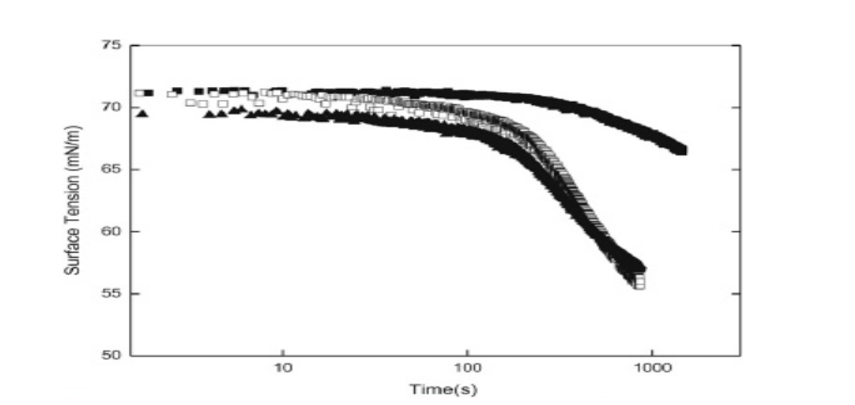
Figure 2: Dynamic surface tension of gum Arabica; Source: (Cao, et al., 2013)
As explained by Tradelink, et al., (2017), hydrophobicity is illustrated as the molecule’s physical property which does not show attraction towards a mass of water.
Gum Arabica has a complex mixture of biopolymers and it has at least three fractions of high molecular weight. It has been suggested that surface – active fraction composes of arabinogalactan blocks which are linked to polypeptide chain.
According to McClements, (2009), the capability of gum Arabica to get adsorbed into lipid surfaces is due to this chain of polypeptide comprises of hydrophobic side groups.
Whereas, Chen, (2015) has suggested that the arabinogalactan blocks which are hydrophilic in nature gets into aqueous phase. The interfacial film developed by gum Arabica has developed a property of stability with relation to droplet aggregation which is normally done through steric repulsion.
The molecular mass of arabino – galectin – protein also surges with the growth of tree.
Research gap
The research study outlines the factors such as temperature, molecular weight, viscosity, hydrophobicity, surface tension and pH of gum Arabica which impact the adsorption in an oil and water emulsion.
As the literature review is constructed on the basis of the objectives and aims developed in the first chapter of the study, focus has been given to justify the aims and objectives through contents from different secondary sources.
But while performing the research, certain gaps have come into the picture.
Information collected from these various secondary sources were not conclusive enough to explain the factors impacting the adsorption of emulsifier like gum Arabica into the oil phase.
Less involvement of quantitative approach can be seen as another research gap of this chapter. Therefore, other methods such as quantitative approach is required to be prioritized in order to establish a more conclusiveness of the study.
Summary
This chapter focuses on different aspects of gum Arabica and its application as an emulsifier.
This chapter also illustrates the characteristics of acacia family and the factors such as temperature, molecular weight, viscosity, hydrophobicity, surface tension and pH of gum Arabica which impact the adsorption into the oil phase.
The chapter has also shown light on the concept of emulsion and the properties of emulsion suggests that it appears to be white or cloudy when formed due to the fact that the light is being scattered off from the interface layer of two liquids.
Therefore, concentration of the emulsion explains its color. The emulsion even appears as yellow as the distorted color would lead to longer wavelength when the emulsion is highly concentrated.
The application of the emulsifiers have been broadly discussed as they are usually described as surfactants which can be further categorized as hydrophilic and lipophilic.
Emulsifiers avoid the oil and water mixture from coalescing by developing barriers around the droplets helps to create stable emulsion. In addition of making stable emulsion, these emulsifiers have the capabilities to react with food ingredients.
Highly soluble in water characteristics explains its applications in different sectors and it is also used as an aerating agent, crystallization inhibitor and even starch complexing agent.
Gum acacia is also considered as natural gum which comprises of hardened sap of the different acacia species and it is a mixture of polysaccharides and glycoproteins.
Gum Arabica is colorless and odorless. Glycoprotein, arabino – galectin and arabino – galectin – protein are the three main component of Gum acacia.
In order to create an emulsion, the concentration of gum Arabica required would be higher than what is need for creation of pure protein as the proteinaceous component of gum Arabica is only used in emulsion.
The fact that it is edible and soluble in water and thus finds its application as emulsion stabilizer in food and beverage sector.
Gum Arabica has found its application in ceramic industry due to its role as binder which adheres glaze and clay together prior being fired.
It also performs the role of a deflocculant and thereby improves the fluidity of the mixture. On the other hand, acacia family comprises of more than 800 species of shrubs and trees of the family of pea.
These shrubs and trees are found in subtropical and tropical regions across the globe, especially in Africa, Asia, America and Australia.
The major impact of the temperature is seen on how it regulates the viscosity of the emulsions as increase in the temperature leads to the decrease in viscosity.
Increase in temperature also mitigates the interfacial viscosity which eventually leads to faster drop coalescence and rate of film – drainage.
The viscosity of the gum solutions falls down when come in contact with the electrolytes because of the charge screening and undissociated nature of carboxyl group is observed at low ph.
Compact molecules of the gum Arabica is because of its highly balanced structure extracted from Acacia Senegal and it also creates a small hydrodynamic volume and this has resulted in gum Arabica becoming viscous only when subjected to higher temperatures.
Surface tensions of gum Arabica drops down with the increase in time and normally takes the structure of a plateau. The capability of gum Arabica to get adsorbed into lipid surfaces is due to this chain of polypeptide comprises of hydrophobic side groups.
References
Al-Assaf, S., Phillips, G. & Amar, V., 2009. Handbook of hydrocolloids. 2nd ed. Durban: Gum ghatti.
Anton, N. & Vandamme, T., 2011. Nano-emulsions and micro-emulsions: clarifications of the critical differences. Pharmaceutical research, 28(5), pp. 978-985.
Bico, J., Reyssat, É. & Roman, B., 2018. Elastocapillarity: When surface tension deforms elastic solids. Annual Review of Fluid Mechanics, 50(1), pp. 629-659.
Cao, C., Zhang, L., Zhang, X. & Du, F., 2013. Effect of gum arabic on the surface tension and surface dilational rheology of trisiloxane surfactant. Food hydrocolloids, 30(1), pp. 456-462.
Castellani, O. et al., 2010. Hydrocolloids with emulsifying capacity. Part 2–Adsorption properties at the n-hexadecane–Water interface. Food Hydrocolloids, 24(2-3), pp. 121-130.
Cathles, L., 2015. Viscosity of the Earth’s Mantle. 1st ed. Princeton: Princeton University Press.
Charoen, R. et al., 2011. Influence of biopolymer emulsifier type on formation and stability of rice bran oil‐in‐water emulsions: whey protein, gum arabic, and modified starch. Journal of Food Science, 76(1), pp. 165-172.
Chen, L., 2015. Emulsifiers as food texture modifiers. Modifying Food Texture , 1(1), pp. 27-49.
Cosentino, A., 2015. EFFECTS OF DIFFERENT BINDERS ON TECHNICAL PHOTOGRAPHY AND INFRARED REFLECTOGRAPHY OF 54 HISTORICAL PIGMENTS. International Journal of Conservation Science, 6(3), p. 1.
Cui, S., Wu, Y. & Ding, H., 2013. The range of dietary fibre ingredients and a comparison of their technical functionality. Fibre-Rich and Wholegrain Foods, 1(1), p. 1.
Davidov-Pardo, G., Gumus, C. & McClements, D., 2016. Lutein-enriched emulsion-based delivery systems: influence of pH and temperature on physical and chemical stability. Food chemistry, 196(1), pp. 821-827.
Derkach, S. et al., 2018. Kinetics of crystallization of aqueous droplets in water-in-crude oil emulsions at low temperatures. Energy & fuels, 32(2), pp. 2197-2202.
Dickhout, J. et al., 2017. Produced water treatment by membranes: a review from a colloidal perspective. Journal of colloid and interface science, 487(1), pp. 523-534.
Donsì, F., Sessa, M. & Ferrari, G., 2011. Effect of emulsifier type and disruption chamber geometry on the fabrication of food nanoemulsions by high pressure homogenization. Industrial & Engineering Chemistry Research, 51(22), pp. 7606-7618.
Du, K. et al., 2010. Adsorption energy of nano-and microparticles at liquid− liquid interfaces. Langmuir, 26(15), pp. 12518-12522`.
Fang, Y. et al., 2010. Interaction of gum arabic with fatty acid studied using electron paramagnetic resonance. Biomacromolecules, 11(5), pp. 1398-1405.
Gadhave, A., 2014. Determination of hydrophilic-lipophilic balance value. Int. J. Sci. Res, 3(4), pp. 573-575.
Grdadolnik, J., Merzel, F. & Avbelj, F., 2017. Origin of hydrophobicity and enhanced water hydrogen bond strength near purely hydrophobic solutes. Proceedings of the National Academy of Sciences, 114(2), pp. 322-327.
Ilavarasan, R. & Vadivelu, L., 2017. Phytochemical and Quality Assessment of Acacia nilotica Linn and Acacia leucophloea willd Flowers. Pharmacognosy Journal, 9(6), p. 1.
Kasaai, M., 2018. Molecular Weight Distribution for Biopolymers: A Review. Journal of Polymer and Biopolymer Physics Chemistry, 6(1), pp. 39-44.
Kennedy, J., Phillips, G. & Williams, P. e., 2012. Gum arabic. 1st ed. London: Royal Society of Chemistry.
Kiiru, S., Mahungu, S. & Omwamba, M., 2018. Preparation and analysis of goat milk mozzarella cheese containing soluble fiber from Acacia senegal var. kerensis. African Journal of Food Science, 12(3), pp. 46-53.
Liu, S., Elmer, C., Low, N. & Nickerson, M., 2010. Effect of pH on the functional behaviour of pea protein isolate–gum Arabic complexes. Food Research International, 43(2), pp. 489-495.
McClements, D., 2009. Biopolymers in food emulsions. Modern biopolymer science, 1(1), pp. 129-166.
McClements, D., 2010. Emulsion design to improve the delivery of functional lipophilic components. Annual review of food science and technology, 1(1), pp. 241-269.
Muhammad, Z. et al., 2016. Density and taxonomic diversity of understory vegetation in relation to site conditions in natural stands of Acacia modesta in Malakand Division, Khyber Pakhtunkhwa, Pakistan. Science, 35(1), pp. 26-34.
Mujawamariya, G., Burger, K. & D’Haese, M., 2012. Quality of gum arabic in Senegal: Linking the laboratory research to the field assessment. Quarterly Journal of International Agriculture, 51(892-2016-65172), p. 357.
Ngorima, A. & Shackleton, C., 2019. Livelihood benefits and costs from an invasive alien tree (Acacia dealbata) to rural communities in the Eastern Cape, South Africa. Journal of environmental management, 229(1), pp. 158-165.
Niu, F. et al., 2016. Ovalbumin/gum arabic-stabilized emulsion: Rheology, emulsion characteristics, and Raman spectroscopic study. Food Hydrocolloids, 52(1), pp. 607-614.
Shi, H. et al., 2019. Surface sediments formation during auto-hydrolysis and its effects on the benzene-alcohol extractive, absorbability and chemical pulping properties of hydrolyzed acacia wood chips. Bioresource technology, 1(1), p. 121604.
Tadros, T. e., 2013. Emulsion formation and stability. 1st ed. London: John Wiley & Sons.
Zeeb, B., Saberi, A., Weiss, J. & McClements, D., 2015. Retention and release of oil-in-water emulsions from filled hydrogel beads composed of calcium alginate: impact of emulsifier type and pH. Soft Matter, 11(11), pp. 2228-2236.
Zhang, X., Wu, J. & Niu, J., 2016. PCM-in-water emulsion for solar thermal applications: The effects of emulsifiers and emulsification conditions on thermal performance, stability and rheology characteristics. Solar Energy Materials and Solar Cells, 147(1), pp. 211-224.
Know more about UniqueSubmission’s other writing services:
Assignment Writing Help
Essay Writing Help
Dissertation Writing Help
Case Studies Writing Help
MYOB Perdisco Assignment Help
Presentation Assignment Help
Proofreading & Editing Help